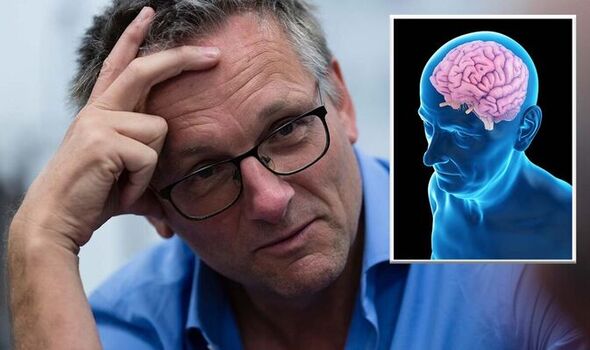
The revolutionary medication is being celebrated as the harbinger of the conclusion to the war against Alzheimer’s disease.
Projections from a leading expert suggest that individuals might have access to this groundbreaking Alzheimer’s treatment as early as the forthcoming year. However, scientists are cautioning that significant overhauls are imperative within the dementia services in the UK to effectively administer the drug, pending regulatory approval. They emphasize the necessity for extensive changes in clinics to accommodate the roughly five percent of patients eligible for this game-changing drug.
Known as Lecanemab, the drug has exhibited the ability to purge the brain of toxic amyloid protein and defer the onset of symptoms during clinical trials. This achievement solidifies its status as the world’s premier treatment capable of impeding cognitive decline.
Renowned geneticist Professor Sir John Hardy is optimistic about a new era of treatments focusing on amyloid, though he, alongside others, warns about the scarcity of clinics in the UK for early disease diagnosis. He stresses the urgency of significantly reducing NHS waiting times to avoid patients missing out on potential benefits.
Speaking at a Science Media Centre event in London, Sir John expressed his enthusiasm, stating, “I’m really excited about the work that’s going to be presented because we’ve seen press releases, and that’s a little bit dangerous. I actually think this is a historic moment. It’s taken a long time to get here, and we first suggested amyloid therapies in 1992.”
Results from a Phase 3 trial of the monoclonal antibody treatment, published in September, demonstrated its promise. The drug, tested on 1,795 patients across the US, Japan, Europe, and China, exhibited a 27 percent reduction in the rate of cognitive decline after 18 months compared to a placebo, according to manufacturers Eisa and Biogen.
The trial used a scale evaluating various cognitive domains, including memory, orientation, judgment, problem-solving, community affairs, home, hobbies, and personal care. A notable side effect observed was brain swelling, with a rate of 12.5 percent for Lecanemab patients compared to 1.7 percent in the placebo group. The companies, however, deemed these effects “within expectations.”
The complete trial data is slated to be presented at a major Alzheimer’s conference on November 29 and subsequently published this year in a peer-reviewed journal.
While not presenting a cure, this discovery offers the potential to enhance the quality of life for millions of individuals currently grappling with neurodegenerative diseases in the UK.
Addressing a pre-conference briefing, Dr. Susan Kohlaas, director of research at Alzheimer’s Research UK, emphasized the urgency brought by the Lecanemab results in improving Alzheimer’s disease diagnoses. Dementia diagnosis rates, hindered by the pandemic, hover around 62 percent, below the national target of 66.7 percent. Dr. Liz Coulthard, associate professor in dementia neurology, highlights the recent availability of biomarker tests for accurate Alzheimer’s diagnoses, a significant advancement in the last five years.
RELATED ARTICLES
- EVs pollute 1850 times more than Fossil Fuel cars according to new study
- UK Warns that China is Preparing for Total Nuclear War with the West
- Jacob Rothschild Dies At Age 87
- UK Government-Funded Study Found Virtually No Dental Benefit From Fluoridation
- UK Elderly Couple Ordered in a Council Letter to Sell Their House as its Needed to House Migrants










The new drug is not worth anyone losing their minds over.